 |
 |
 |
 |
 |
 |
 |
 |
|
High-Pressure Synthesis of Deep Mantle Minerals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deep mantle minerals (high-pressure minerals; wadsleyite, hydrous wadsleyite, ringwoodite,
hydrous ringwoodite, bridgmanite, majorite, etc.) have been synthesized
for mineral-physics experiments by high-pressure synthesis using a Kawai-type multianvil apparatus. Recently I succeeded to synthesize huge single crystals (0.5-1 mm) of wadsleyite (Kawazoe et al., 2015) and ringwoodite. Target properties (with collaborations) are: ・Sound velocity (Brillouin spectroscopy, ultrasonic interferometry) ・Crystal structure (single-crystal X-ray diffraction) ・Atomic diffusivity (atomic diffusion experiments) ・Thermal conductivity (thermoreflectance technique) ・Rheological properties (deformation experiments) ・Topotaxy in transformation (transmission electron microscopy), etc. In particular, single crystals are useful to study orientation-dependent properties of the deep mantle minerals (all of the above properties). Some crystals are cut with focused iron beam (FIB) to perform high-pressure experiments with a diamond anvil cell (DAC) (Marquardt and Marquardt, 2012). In addition, the huge wadsleyite single crystals is going to be used in a multianvil apparatus. |
 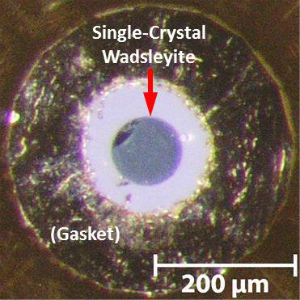 |
| Fig. (Upper) Deep mantle minerals (high-pressure minerals) synthesized by high-pressure synthesis using the Kawai-type multianvil apparatus (wadsleyite, ringwoodite, bridgmanite and majorite). Natural olivine is also shown. (Lower) Single-crystal wadsleyite loaded in a diamond anvil cell (DAC) (courtesy of Johannes Buchen, Drs. Hauke Marquardt and Katharina Marquardt). |
|
1. Bridgmanite (Mg Silicate Perovskite)Bridgmanite is the most dominant mineral at the lower mantle (and the whole mantle). |
|
 |
 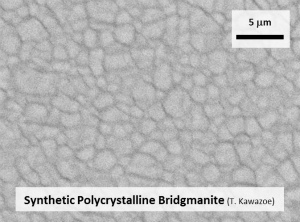 |
| Fig. Fe-Al-bearing bridgmanite synthesized by high-pressure synthesis using the Kawai-type multianvil apparatus. (Left) Single-crystal bridgmanite. (Upper right) Polycrystalline bridgmanite. (Lower right) A backscattered electron image of the polycrystalline bridgmanite (courtesy of Dr. Ryosuke Sinmyo). |
|
2. RingwooditeRingwoodite is the most dominant mineral at the lower part of the mantle transition zone (~520-660 km depth).Ringwoodite is also called as γ-(Mg,Fe)2SiO4 (Morimoto et al., 1970) or silicate spinel phase. Synthetic ringwoodite (ahrensite) was synthesized in Fe2SiO4 composition for the first time in 1958 (Ringwood, 1958b). Natural ringwoodite was discovered in the Tenham meteorite in 1969 (Binns et al., 1969). (More information about high-pressure minerals in shocked meteorites can be found at Dr. Naotaka Tomioka's weg site.) Dislocations and stacking faults in ringwoodite were characterized for the first time in 1980 (Madon and Poirier, 1980). |
|
  |
  |
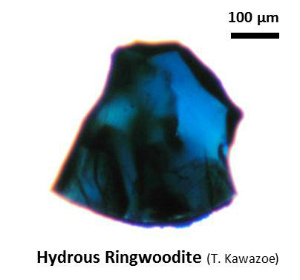
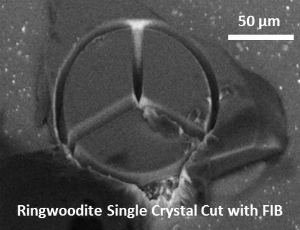
| Fig. Ringwoodite synthesized by high-pressure synthesis using the Kawai-type multianvil apparatus. (Upper left) Hydrous Fe-bearing ringwoodite (up to ~0.5 mm; synthesized from olivine with distilled water in a welded Pt capsule). (Right) Fe-bearing ringwoodite (synthesized from olivine with no additional water). (Lower left) Ringwoodite single crystal cut with focused ion beam (FIB) (courtesy of Kirsten Schulze, Drs. Hauke Marquardt and Katharina Marquardt). |
|
| The crystal structure of ringwoodite (γ-(Mg,Fe)2SiO4) belongs to the cubic crystal system. The crystal structure of ringwoodite is the normal spinel structure (Brown, 1982). The space group of the ringwoodite structure is Fd-3m. Ringwoodite is optically isotropic. Among crystals with the spinel structure, crystals with the chemical formula of M2SiO4 are classified as silicate spinel (M = divalent cation). The silicate spinel includes Mg2SiO4 (Sasaki et al., 1982), Fe2SiO4 (Yagi et al., 1974), Co2SiO4 (Morimoto et al., 1974), Ni2SiO4 (Yagi et al., 1974). In right figures, SiO4 tetrahedra (blue) are shown with MO6 octahedra (brown), Mg-Fe atoms (orange) and O atoms (red). O sub-lattice of the ringwoodite structure is the slightly distorted cubic close-packed (CCP) array, which is identical to the face-centered cubic (FCC) structure. The unit cell of the FCC/CCP structure contains 8 tetrahedral and 4 octahedral positions. The unit cell of the spinel structure consists of 8 unit cells of the FCC/CCP structure. Therefore, the unit cell of the spinel structure contains 64 tetrahedral and 32 octahedral positions. The unit cell contains eight formula units (Z = 8). This means that the unit cell contains 32 O atoms, 8 Si atoms and 16 Mg-Fe atoms. Mg-Fe atoms occupy 1/2 of the octahedral positions, and Si atoms occupy 1/8 of the tetrahedral positions. SiO4 tetrahedra are isolated in ringwoodite while wadsleyite has Si2O7 group. Distortion of the O sub-lattice in the spinel structure is expressed by the u parameter, which is the position parameter of O atoms. The u parameter is 0.375 (= 3/8) when the O sub-lattice is the ideal CCP-FCC structure. When the u parameter is smaller than 0.375, the tetrahedral site favors relatively small cations. In addition, shared edges between octahedra are longer than unshared edges. The u parameters of Mg2SiO4 and Fe2SiO4 ringwoodite are 0.3685 (Sasaki et al., 1982) and 0.3658 (Yagi et al., 1974). The u parameter of Co2SiO4 spinel is 0.3666 (Morimoto et al., 1974). The close-packed planes of the O sub-lattice are the four {111} planes. The close-packed directions of the O sub-lattice are the <110> directions. |
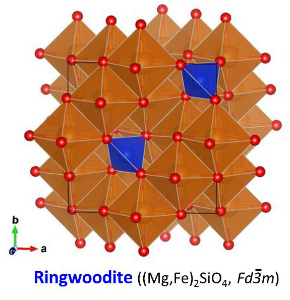 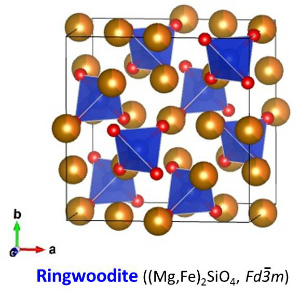 |
| Fig. The crystal structure of ringwoodite (Hazen et al., 1993). | |
3. Ringwoodite - WadsleyiteThe 520-km seismic discontinuity is attributed to a structural phase transformation from wadsleyite to ringwoodite. |
|
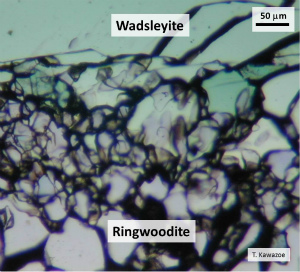 |
Crystal structures of ringwoodite, wadsleyite and olivine were discussed based on differences in stacking of bands composed of octahedra and tetrahedra (Morimoto et al., 1974). |
| Fig. Simulated 520-km discontinuity, where ringwoodite coexists with wadsleyite at ~18 GPa and ~1800 K at boundary of wadsleyite-ringwoodite transition. Wadsleyite and ringwoodite regions correspond with the upper and lower parts of the mantle transition zone, respectively. | |
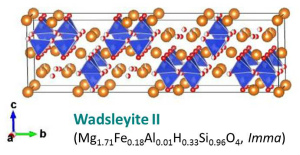 |
Wadsleyite II (Smyth and Kawamoto, 1997) may be an intermediate phase between wadsleyite and ringwoodite in MgO-FeO-Fe2O3-SiO2-H2O system (Smyth et al., 2005). Wadsleyite II is not stable in Mg2SiO4 (Tokar et al., 2013). |
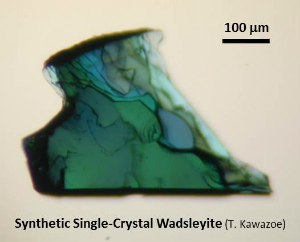
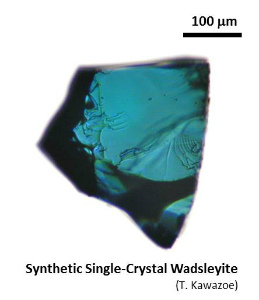
4. WadsleyiteWadsleyite is the most dominant mineral at the upper part of the mantle transition zone (~410-520 km depth).Wadsleyite is also called as β-(Mg,Fe)2SiO4 (Morimoto et al., 1970) or modified spinel phase. Synthetic wadsleyite was synthesized for the first time in 1966 (Ringwood and Major, 1966). Natural wadsleyite was discovered in the Tenham meteorite in 1979 (Putnis and Price, 1979). (More information about high-pressure minerals in shocked meteorites can be found at Dr. Naotaka Tomioka's weg site.) Hydrous wadsleyite was predicted in 1987 (Smyth, 1987) and was confirmed in 1991 (McMillan et al., 1991). |
|
   |
   |
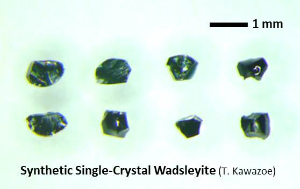
| Fig. Wadsleyite synthesized by high-pressure synthesis using the Kawai-type multianvil apparatus. (Left) Fe-bearing single-crystal wadsleyite (up to ~1.2 mm, Kawazoe et al., 2015). (Upper right) Hydrous Fe-bearing wadsleyite single crystal. (Middle right) Polycrystalline wadsleyite (e.g. Kawazoe et al., 2010 JGR). (Lower right) Fe-bearing single-crystal wadsleyite loaded in a diamond anvil cell (DAC) for Brillouin spectroscopy (courtesy of Johannes Buchen, Drs. Hauke Marquardt and Katharina Marquardt). |
|
The crystal structure of (water-poor) wadsleyite belongs to the orthorhombic crystal system (Mg2SiO4: Horiuchi and Sawamoto, 1981; (Mg,Fe)2SiO4: Sawamoto and Horiuchi, 1990). The crystal structure of water-rich wadsleyite belongs to the monoclinic crystal systems (Smyth et al., 1997; Kudoh and Inoue, 1999). The crystal structure of wadsleyite is the wadsleyite structure and identical to that of the spinelloid III structure. The space groups are Imma and I2/m for water-poor and water-rich wadsleyite, respectively (Smyth et al., 1997). Wadsleyite is optically anisotropic. In right figures, SiO4 tetrahedra (blue) are shown with MO6 octahedra (brown), Mg-Fe atoms (orange) and O atoms (red). O sub-lattice of the wadsleyite structure is the slightly distorted cubic close-packed (CCP) arrary, which is identical to the face-centered cubic (FCC) structure. There are eight formula units per unit cell (Z = 8). This means that the unit cell contains 32 O atoms, 8 Si atoms and 16 Mg-Fe atoms. There are three octahedral cation site, M1, M2, and M3, and four O positions, O1, O2, O3, and O4. Mechanism of hydration of wadsleyite is protonation of O1 charge-balanced by octahedral vacancy at M3 (Smyth et al., 1997). A proton position is primarily on the O1-O4 edge of the M3 octahedron (Jacobsen et al., 2005; Sano-Furukawa et al., 2011) Coupling of Fe3+ and H+ substitutes for Si4+ in hydrous wadsleyite (Bolfan-Casanova et al., 2012; Smyth et al., 2014). Si2O7 group in wadsleyite is unique feature comparing isolated SiO4 tetrahedra in olivine and ringwoodite. Longer b suggests that the wadsleyite (010) plane is a strong candidate for the slip plane of the easiest slip system (e.g. [u0w](010)). |
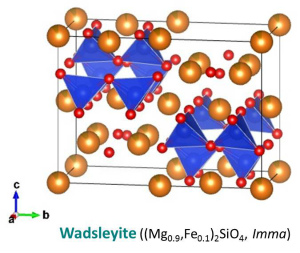 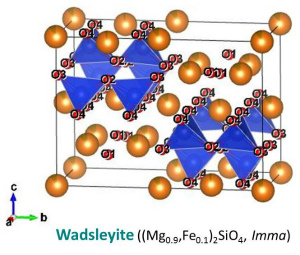 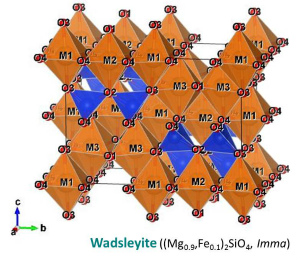 |
| Fig. Crystal structure of wadsleyite (Sawamoto and Horiuchi, 1990). Each site of O atoms are labeled in the lower figure. | |
5. Majorite (Majoritic Garnet)Majorite is the second dominant mineral at the mantle transition zone (~410-660 km depth).(More information about high-pressure minerals in shocked meteorites can be found at Dr. Naotaka Tomioka's weg site.) |
|
 |
 |
| Fig. Fe-Al-bearing polycrystalline majorite synthesized by high-pressure synthesis using the Kawai-type multianvil apparatus. | |
II. High-Pressure Synthesis
| I have used a Kawai-type multianvil apparatus for the high-pressure synthesis of the deep mantle minerals. This apparatus is suitable for our purpose because of relatively large sample volume, available pressure-temperature conditions and homogeneity in pressure and temperature in a sample. Please go to the page introducing a multianvil apparatus for technical details. | |
 |
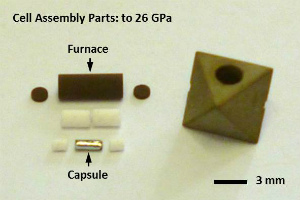 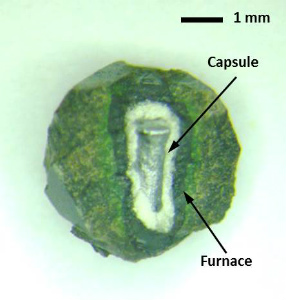 |
| Fig. (Left) A Kawai-type multianvil apparatus (Bayerisches Geoinstitut, University of Bayreuth). (Upper right) Cell assembly parts for high-pressure synthesis to 26 GPa. (Lower right) A recovered cell assembly after synthesis of Fe-Al-bearing bridgmanite at 26 GPa. |
|
III. Natural and Synthetic Materials
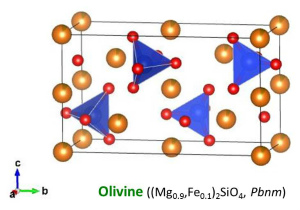
1. OlivineOlivine is the most dominant mineral at the upper mantle.Natural olivine is used as a starting material for the high-pressure synthesis of wadsleyite and ringwoodite. |
|
  |
 |
| Fig. Single-crystal olivine. (Upper left) An olivine single crystal from San Carlos. (Right) A San Carlos olivine single crystal shaped as cuboid for starting material of experiments. (Lower left) Crystal structure of olivine (McCormik et al., 1987). SiO4 tetrahedra (blue) are shown with Mg-Fe (orange) and O (red) atoms. SiO4 tetrahedra are isolated in olivine while wadsleyite has Si2O7 group. |
|
2. Orthopyroxene |
|
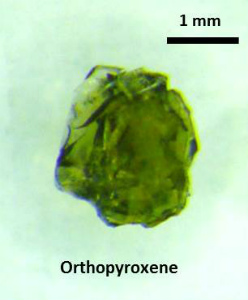 |
Fig. Single-crystal orthopyroxene from a San Carlos xenolith (courtesy of Dr. Robert Farla). Orthopyroxene is used as starting material for the high-pressure synthesis of Fe,Al-bearing bridgmanite and majorite. |
3. Glass |
|
SiO3-EN 300px.jpg) |
Fig. Glass spheres with MgSiO3 and (Mg,Fe)SiO3 compositions (courtesy of Dr. Sylvain Petitgirard). The glass will be used as starting materials for the high-pressure synthesis of bridgmanite and majorite. |
4. Shocked Meteorite |
|
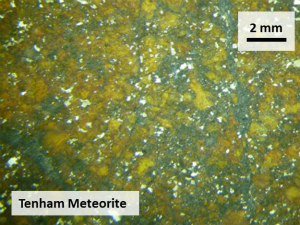 Fig. Tenham meteorite. Ringwoodite was found in the Tenham meteorite for the first time and was named after Prof. A. Ringwood (Binns et al., 1969). Wadsleyite was also discovered in the Tenham meteorite for the first time (Putnis and Price, 1979). Bridgmanite was found in the Tenham meteorite for the first time (Tomioka and Fujino, 1997). Majorite and akimotoite were also found in this meteorite. |
 Fig. Tissint martian meteorite. |
5. Peridotite Xenolith |
|
 Fig. A peridotite xenolith from Ichinomegata, Japan. This rock is mainly composed of olivine, orthopyroxene and clinopyroxene. |
|
6. Eclogite |
|
 Fig. Eclogite from Higashi-Akaishi, Ehime, Japan. Large crystals are garnet. |
IV. Crystal Structure
1. Crystal Structure Drawing |
|
| The crytal structures were drawn with VESTA (Momma and Izumi, 2011). |
|
2. Crystal Structure Data |
|
| Crystal structure data were downloaded at the American Mineralogist Crystal Structure Database |
Copyright (c) Since 2012. Takaaki KAWAZOE. All Rights Reserved.